2. Chemistry Acid base reactions
See: https://mirror.cse.unsw.edu.au/pub/CTAN/macros/latex/contrib/mhchem/mhchem.pdf
The mhchem package makes it easy to write acid base reactions.
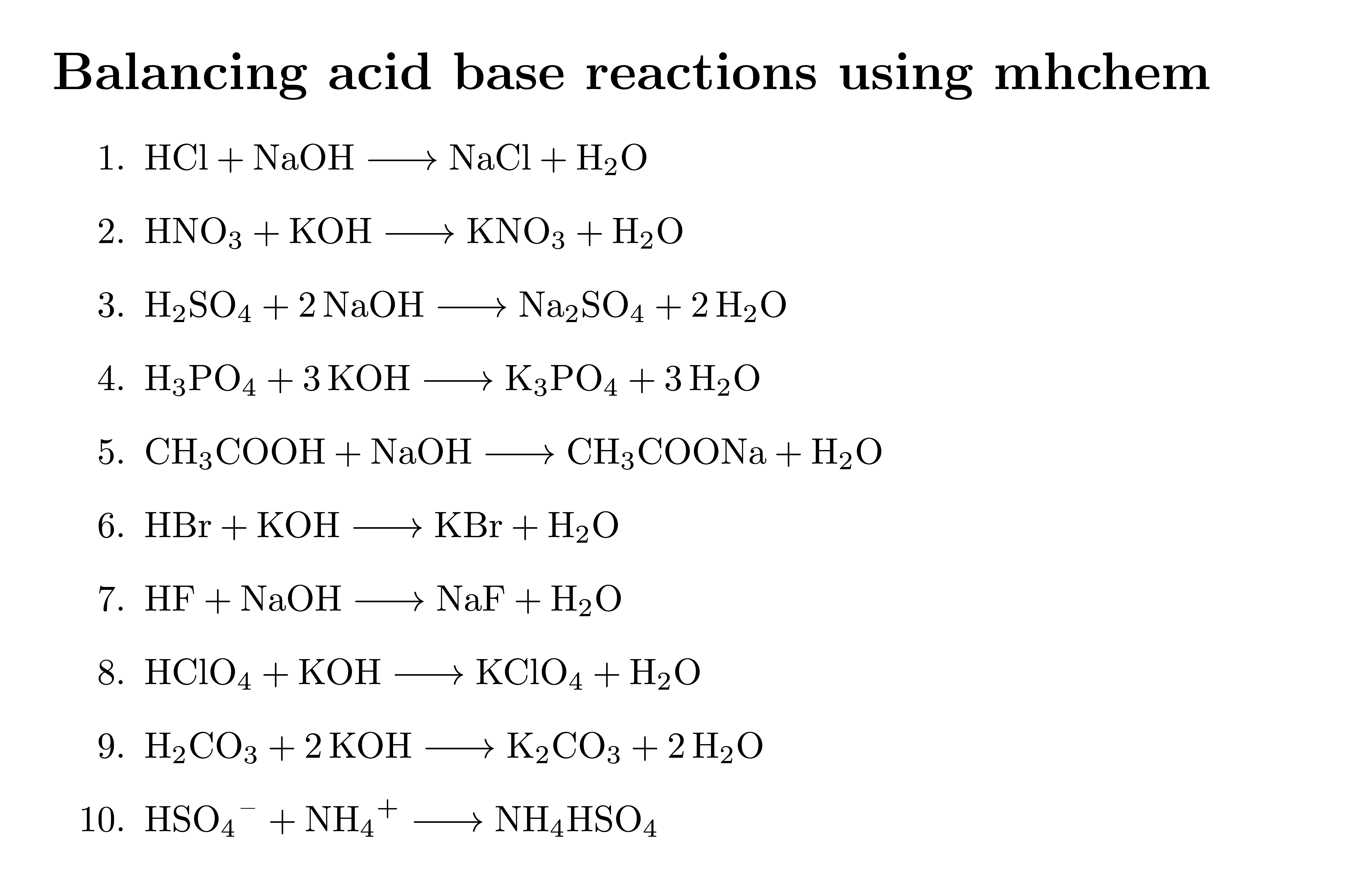
1\documentclass[border=5mm]{standalone}
2\usepackage[version=4]{mhchem}
3\begin{document}
4\begin{minipage}{\textwidth}
5
6\section*{Balancing acid base reactions using mhchem}
7
8\begin{enumerate}
9 \item \ce{HCl + NaOH -> NaCl + H2O}
10 \item \ce{HNO3 + KOH -> KNO3 + H2O}
11 \item \ce{H2SO4 + 2NaOH -> Na2SO4 + 2H2O}
12 \item \ce{H3PO4 + 3KOH -> K3PO4 + 3H2O}
13 \item \ce{CH3COOH + NaOH -> CH3COONa + H2O}
14 \item \ce{HBr + KOH -> KBr + H2O}
15 \item \ce{HF + NaOH -> NaF + H2O}
16 \item \ce{HClO4 + KOH -> KClO4 + H2O}
17 \item \ce{H2CO3 + 2KOH -> K2CO3 + 2H2O}
18 \item \ce{HSO4- + NH4+ -> NH4HSO4}
19\end {enumerate}
20\end {minipage}
21\end {document}